VEGFR2
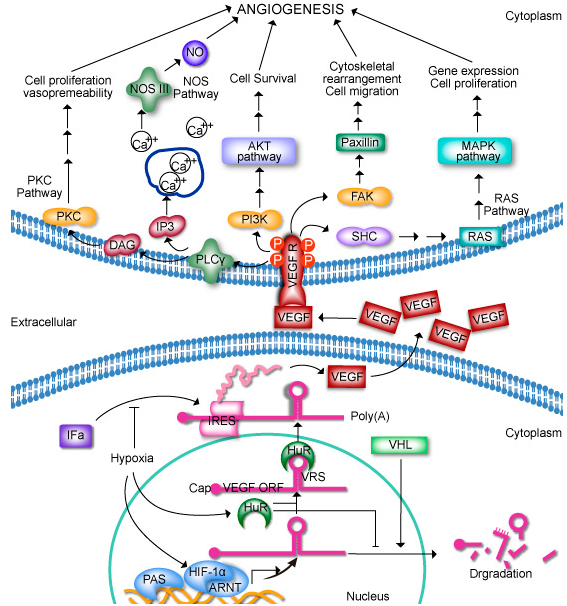
Vascular endothelial growth factors (VEGFs) are crucial regulators of vascular development during embryogenesis as well as blood-vessel formation (angiogenesis) in the adult. In mammals, five VEGF ligands, which occur in several different splice variants and processed forms, have been identified so far. These ligands bind in an overlapping pattern to three receptor tyrosine kinases (RTKs), known as VEGF receptor-1, -2 and -3 (VEGFR1–3). In certain respects, VEGFs share regulatory mechanisms with other well-characterized RTKs, such as the platelet-derived growth-factor receptors (PDGFRs) and the epidermal growth-factor receptors (EGFRs).These mechanisms include receptor dimerization and activation of the tyrosine kinase, as well as creation of docking sites for signal transducers. Moreover, the VEGFRs induce cellular processes that are common to many growth-factor receptors, including cell migration, survival and proliferation.
VEGFR2 (vascular epithelial growth factor receptor 2, or KDR) is the receptor of VEGF (and its isoforms) which mediates normal endothelial cell survival, migration, and proliferation of blood vessels. The important role of VEGFR2 signaling during development and in neovascularization in physiological or pathological conditions has allowed the design of clinically beneficial therapies.
Breast cancers can express vascular endothelial growth factor (VEGF), or VEGFR2 and increased VEGF expression in breast cancers is associated with tumor progression and increased risk of recurrence.
VEGFR2 is often overexpressed in metastatic colorectal cancer and correlated with increased tumor size and dedifferentiation of cancer cells.
VEGFR2 along with other genes comprise a prognostic gene set for disease outcome of lung cancer, used as a marker for efficacy to treatment with anti-angiogenic agents (Bevacizumab).


